Quality Systems & Good Distribution Practices
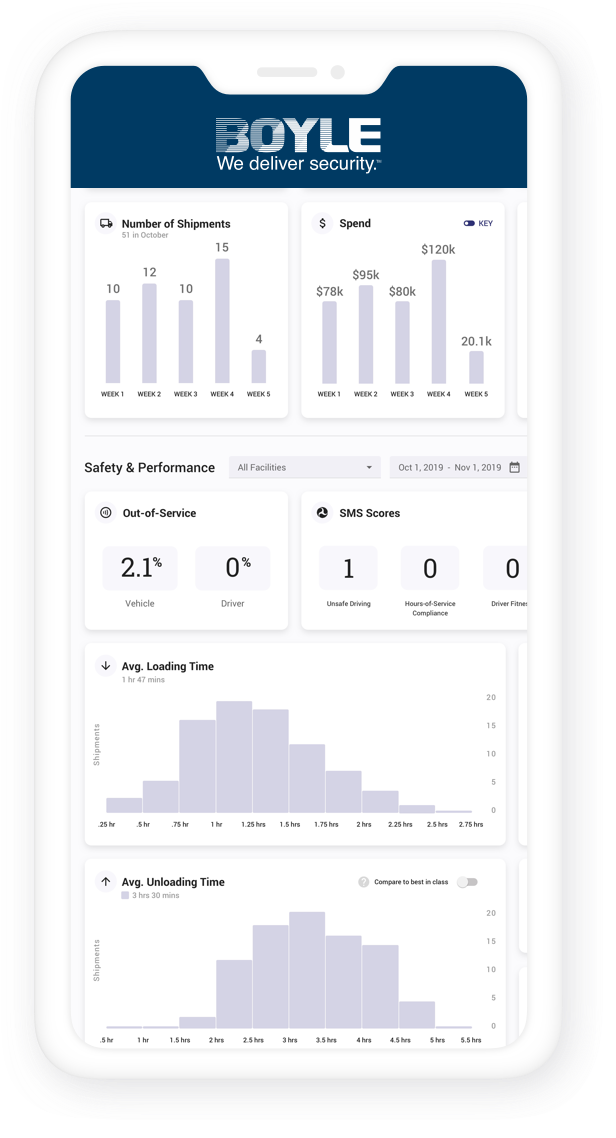
One thing common to the aerospace and defense and pharmaceutical industries is a long history of the most stringent quality systems and SOP-driven operations you will find anywhere. We have consistently mirrored this since our inception, and we are regularly acknowledged by the defense and pharmaceutical industries for our quality system and overall approach. In addition to routine audits by customers and the Department of Defense, we strictly adhere to Good Distribution Practices (GDP) and have been recognized by the National Defense Transportation Association (NDTA) with its Distinguished Service Award.
Our quality management system has been ISO-certified since 1997. Not only do we perform internal audits, but we also undergo regularly scheduled, rigorous quality audits by our pharmaceutical and government clients.